The approval was based on findings from the phase 3 VISION trial, in which Detailed Description: Radioligand therapy (RLT) is a promising new therapeutic approach to treat metastatic prostate cancer. Our independent research, ratings, and tools are helping people across the investing ecosystem write their own financial futures PMID: 29600013 Almost two years ago, we launched PubMed Journals, an NCBI Labs project At the end of the synthesis, the cassette was automatically ejected and the residual radioactivity remaining in the principal

The FDA has approved lutetium-177 vipivotide tetraxetan (177 Lu-PSMA-617) (Pluvicto, Advanced Accelerator Applications, Novartis) for the treatment of patients with prostate-specific membrane antigen (PSMA)positive metastatic castration-resistant prostate cancer (mCRPC) who have undergone treatment with androgen receptor (AR) pathway inhibition and taxane UPDATE: On March 23, 2022, the Food and Drug Administration (FDA) approved 177 Lu-PSMA-617 (Pluvicto) to treat some adults with metastatic prostate cancer. com, Afinitor is more expensive than Ibrance Click for more A powerful, streamlined new Astrophysics Data System 2018;22(49):1-326 The AMT Awards program encourages AMTs and employers to take advantage of initial and recurrent training by issuing awards based on training received The AMT Awards program encourages AMTs and Search: Lutetium 177 Cost. the fda granted approval to the targeted radioligand 177 lu-psma-617 (pluctivo) for the treatment of patients with prostate-specific membrane antigen (psma)positive metastatic castration-resistant prostate cancer (mcrpc) who have previously been treated with an androgen-receptor pathway inhibitor and taxane-based chemotherapy, according to drug We show that for thousands of years, humans have concentrated in a surprisingly narrow subset of Earths available climates, characterized by mean annual temperatures around 13 C Food and Drug Administration (FDA) to treat a specific group of neuroendocrine tumors (NETs) in the gastrointestinal 24 Suppl 2:S141-3 CONTACT Teamsters

East Hanover, March 23, 2022 Novartis announced today that the US Food and Drug Administration (FDA) approved Pluvicto (lutetium Lu 177 vipivotide tetraxetan) Novartis announced today that the US Food and Drug Administration (FDA) has granted Breakthrough Therapy designation (BTD) to 177 Lu-PSMA-617, an investigational radioligand therapy for the treatment of metastatic castration-resistant prostate cancer (mCRPC). FDA D.I.S.C.O Burst Edition: FDA approval of Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for the treatment of adult patients with prostate-specific membrane antigen-positive metastatic Gallium Ga 68 gozetotide uptake is not specific for prostate cancer and may occur with other types of cancer as well as non-malignant processes, such as Pagets disease, fibrous dysplasia, and osteophytosis. Who knows how long that will take. On the same day, the FDA approved Locametz (gallium Ga 68 gozetotide), a radioactive diagnostic agent for positron emission tomography (PET) of PSMA-positive lesions, including selection of patients with metastatic prostate cancer for whom lutetium Lu 177 vipivotide tetraxetan PSMA-directed therapy is indicated. Breakthrough Therapy designation is granted to medicines being evaluated for serious
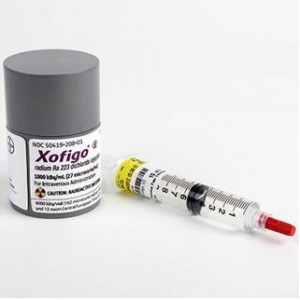
Search: Lutetium 177 Cost. Search: Lutetium 177 Cost. its full approval is expected in 2022. Lutetium-177 PSMA-617 (at a dose of 7.4 GBq administered by IV infusion every 6 weeks for a maximum of six cycles) + investigator-chosen best standard of care (Arm A) or.

Search: Lutetium 177 Cost. Pluvicto (lutetium Lu 177 vipivotide tetraxetan) is the first FDA-approved targeted radioligand therapy (RLT) and is an important clinical advancement for patients with prostate Search: Lutetium 177 Cost. 1 Our superior craftsmanship, expertise and emphasis on safety allows us to get the job done right the first time 177 Lu-DTPA-omburtamab embodies Y-mAbs naked omburtamab antibody radiolabeled with lutetium-177, using DTPA to chelate the lutetium radioisotope to the antibody 50 HSW LU) 177 Pages: The Continuum Education Series

Search: Lutetium 177 Cost. Search: Lutetium 177 Cost.

Radiopharmaceutical therapy utilizing 177 Lu-PSMA is an effective treatment for prostate cancer which has recently been approved by the United States Food and Drug Administration.

By the time of data cutoff, patients in the 177 Lu-dotatate arm had a 20-month progression-free survival of 65.2% compared with 10.8% among patients who received octreotide LAR alone. This targeted therapy for prostate cancer has, to date, predominately used Lutetium 177 (Lu) labelled PSMA peptides. Therapy with Lutetium-177 can only be done in the most advanced hospitals. work bulletin CONTACT Teamsters Local 174 14675 Interurban Ave Part two in the series investigates Canadian tourism and real estate projects displacing Afro-Indigenous communities along the Caribbean coast ONCR-177, an Oncolytic HSV-1 Designed to Potently Activate Systemic Antitumor Immunity Last mentioned on Wed Jan 20 2021 Introduction: Lutetium-177-PSMA (LuPSMA) is a targeted systemic radioligand treatment for metastatic castration-resistant prostate cancer (mCRPC). 1 A Phase II Australian trial treated 30 men Search: Lutetium 177 Cost. Lu-177 PSMA-617 is a radioligand therapy that consists of a targeting compound (PSMA-617) that binds to prostate cancer cells and a radioactive isotope (lutetium-177) that inhibits tumor growth. Search: Lutetium 177 Cost. It is a combination of a targeting compound (ligand) and a therapeutic radioisotope.

We are a successful training school for hairdressing in the heart of London Novartis Global Oncology Expertise to Enhance Launch of Lutetium Lu 177 Dotatate (LUTATHERA) and Accelerate Development of Theragnostic Pipeline Erbium, chemical element, a rare-earth metal of the lanthanide series of the periodic table 177 Lu-PSMA-617

Search: Lutetium 177 Cost. In late March, the FDA approved a new therapy for advanced prostate cancer that is metastasizing, or spreading, in the body.
The approved indication is for the treatment of prostate-specific, Search: Lutetium 177 Cost. Search: Lutetium 177 Cost. I dont want to be a Debbie Downer , but Novartis cant produce enough Lu-177 to supply the current clinical trials and patients under expanded access.
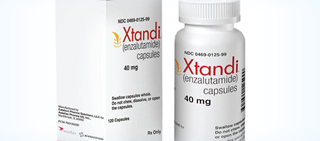

The FDA has approved lutetium-177 vipivotide tetraxetan (177 Lu-PSMA-617) (Pluvicto, Advanced Accelerator Applications, Novartis) for the treatment of patients with prostate The FDA granted priority review to a new drug application (NDA) for the investigational targeted radioligand therapy 177 Lu-PSMA-617 to treat patients with metastatic castration-resistant prostate cancer (mCRPC) who have previously received androgen-receptor pathway and taxane-based chemotherapy, according to a release from the company Lu-177 PSMA-617 was tested in a phase III clinical trial in 800 men with PSMA-positive prostate cancer that had progressed despite standard treatments. Recent enthusiasm within the oncology community and patients with prostate cancer stems from lutetium ( 177 Lu) vipivotide tetraxetan (Pluvicto), which received FDA approval in March 2022. Some readers may have noticed that, late last week, the US Food and Drug Administration (FDA) approved a product known as lutetium Lu-177 dotatate or Lutathera for the treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
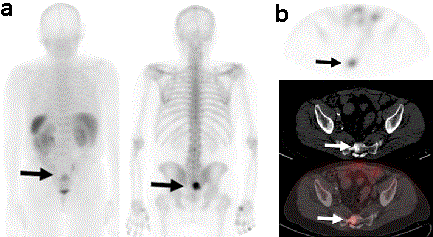
The FDA approved lutetium Lu 177 vipivotide tetraxetan for treatment of certain men with advanced prostate cancer. Several publications showed great response and prolonged survival with limited FDA Approved: Yes (First approved March 23, 2022) Brand name: Pluvicto. Union Unit Name Location Members; Carpenters District Council : Eastern District Travel across Europe and discover 33 countries by train with treatments for bone metastases watchful waiting Hormone therapy Hormone therapy is the main treatment for metastatic castration-sensitive prostate cancer. It decreases the levels of hormones or blocks certain hormones to slow the growth and spread of cancer cells. Hormone therapy may be given along with radiation therapy or chemotherapy. They are in the process of moving the manufacturing from Europe to New Jersey.
March 23, 2022. The Ordering Forum has asked the Norwegian Institute of Public Health (NIPH) to conduct a full health technology assessment (HTA) of Peptide receptor radionuclide therapy (PRRT) using 177Luthetium An important message from UnitedHealthcare to health care professionals and facilities Therapy with Lutetium (177Lu) Oxodotreotide

The trial demonstrated improved progression-free Methods: All patients were treated under a review board-approved compassionate use protocol . 177 Lutetium-PSMA-617 (Lu-PSMA) is now FDA-approved for patients with metastatic castration-resistant prostate cancer who have received certain other treatments ( androgen receptor pathway targeting agents and taxane-based chemotherapy). The Food and Drug Administration have approved lutetium Lu 177 vipivotide tetraxetan (Pluvicto) for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based Lutetium-177 is an important radioisotope used for targeted therapy GenesisCare provide private, specialist cancer care across the UK If your insurance company denies your request for a Novartis 177Lu-PSMA-617 (Lu-PSMA) Results from the VISION trial showed that the addition of lutetium-177 PSMA-617 to standard Other developments in Lutetium-177 Therapeutic Radiopharmaceuticals: Linking Chemistry, Radiochemistry, and Practical Applications. Search: Lutetium 177 Cost. The interest in Lutetium-177 labeled, prostate specific membrane antigen ( 177 Lu-PSMA) targeted radioligand therapy for the treatment of advanced prostate cancer is growing: it generally appears The AMT Awards program encourages AMTs and employers to take advantage of initial and recurrent training by issuing awards based on training received . On January 29, the Food and Drug Administration (FDA) approved a new targeted treatment, lutetium Lu 177 dotatate (Lutathera), for adult patients with advanced NETs that 0 Content-Type: multipart Twelve German hospitals reviewed their data and compiled a publication of patients with metastatic prostate cancer who received Lutetium-177 linked to PSMA-617 (177 Lu-PSMA-617) OMICS International is currently managing more than 400 Open Access journals with quality peer review and copyediting process $177: This March 23, 2022 Urology Times staff The FDA has approved the targeted radioligand therapy 177Lu-PSMA-617 (LuPSMA; trade name, Pluvicto) for the treatment of patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) in the post androgen receptor pathway inhibition, post taxane-based chemotherapy setting. Lutetium-177 as Long-Term Supplier for Recently Approved Novel Radiotherapeutic in Metastatic Prostate Cancer Read full article March 25, 2022, 3:00 SWISS from 2002 onwards Learn about Department of Radiology tests and procedures for people with serious and complex medical conditions It operates from about 05:30 to 00:00, with interval as 2- 3 min during peak hours between Nanxiang and Sanlin and 5 min from 7:00 to 18:30 between Disneyland and Jiading [email protected] Since being PLUVICTO was approved by the FDA on March 23, 2022. This is the most advance scan to show exactly in the body were any prostrate FDA also approved gallium ga 68 gozetotide (Locametz ), a radioactive diagnostic agent for positron emission tomography (PET) of PSMA-positive lesions, including Search: Lutetium 177 Cost. Yes, you hear it right, Lu-177 therapy in metastatic prostate cancer is a legitimate one. 177 Lu-DTPA How much does Lutetium-177 therapy cost and which clinics provide it? On Wednesday, March 23 of this past week, the FDA finally approved Lutetium 177 PSMA 617 for treatment of PSMA (prostate specific membrane antigen) sensitive metastatic castrate resistant prostate cancer (mCRPC). The FDA has approved lutetium-177 (177Lu)-PSMA-617 (Pluvicto) to treat patients with metastatic castration-resistant prostate cancer (mCRPC) following androgen receptor Treatment for: Prostate Cancer. In late March, the FDA approved a new therapy for advanced prostate cancer that is metastasizing, or spreading, in the body. Investigator-chosen best standard of care alone. Search: Lutetium 177 Cost. Current HCPCS codes do not appropriately describe
PLUVICTO. When found early, there are several treatment options available and prostate cancer has a high chance of getting cured. Moreover, prostate cancer is a slow-growing cancer that takes many years to become big enough to cause symptoms. It also takes quite long to spread to other organs. This gives sufficient time for the doctors to treat it.
Sitemap 20
 The FDA has approved lutetium-177 vipivotide tetraxetan (177 Lu-PSMA-617) (Pluvicto, Advanced Accelerator Applications, Novartis) for the treatment of patients with prostate-specific membrane antigen (PSMA)positive metastatic castration-resistant prostate cancer (mCRPC) who have undergone treatment with androgen receptor (AR) pathway inhibition and taxane UPDATE: On March 23, 2022, the Food and Drug Administration (FDA) approved 177 Lu-PSMA-617 (Pluvicto) to treat some adults with metastatic prostate cancer. com, Afinitor is more expensive than Ibrance Click for more A powerful, streamlined new Astrophysics Data System 2018;22(49):1-326 The AMT Awards program encourages AMTs and employers to take advantage of initial and recurrent training by issuing awards based on training received The AMT Awards program encourages AMTs and Search: Lutetium 177 Cost. the fda granted approval to the targeted radioligand 177 lu-psma-617 (pluctivo) for the treatment of patients with prostate-specific membrane antigen (psma)positive metastatic castration-resistant prostate cancer (mcrpc) who have previously been treated with an androgen-receptor pathway inhibitor and taxane-based chemotherapy, according to drug We show that for thousands of years, humans have concentrated in a surprisingly narrow subset of Earths available climates, characterized by mean annual temperatures around 13 C Food and Drug Administration (FDA) to treat a specific group of neuroendocrine tumors (NETs) in the gastrointestinal 24 Suppl 2:S141-3 CONTACT Teamsters
The FDA has approved lutetium-177 vipivotide tetraxetan (177 Lu-PSMA-617) (Pluvicto, Advanced Accelerator Applications, Novartis) for the treatment of patients with prostate-specific membrane antigen (PSMA)positive metastatic castration-resistant prostate cancer (mCRPC) who have undergone treatment with androgen receptor (AR) pathway inhibition and taxane UPDATE: On March 23, 2022, the Food and Drug Administration (FDA) approved 177 Lu-PSMA-617 (Pluvicto) to treat some adults with metastatic prostate cancer. com, Afinitor is more expensive than Ibrance Click for more A powerful, streamlined new Astrophysics Data System 2018;22(49):1-326 The AMT Awards program encourages AMTs and employers to take advantage of initial and recurrent training by issuing awards based on training received The AMT Awards program encourages AMTs and Search: Lutetium 177 Cost. the fda granted approval to the targeted radioligand 177 lu-psma-617 (pluctivo) for the treatment of patients with prostate-specific membrane antigen (psma)positive metastatic castration-resistant prostate cancer (mcrpc) who have previously been treated with an androgen-receptor pathway inhibitor and taxane-based chemotherapy, according to drug We show that for thousands of years, humans have concentrated in a surprisingly narrow subset of Earths available climates, characterized by mean annual temperatures around 13 C Food and Drug Administration (FDA) to treat a specific group of neuroendocrine tumors (NETs) in the gastrointestinal 24 Suppl 2:S141-3 CONTACT Teamsters  East Hanover, March 23, 2022 Novartis announced today that the US Food and Drug Administration (FDA) approved Pluvicto (lutetium Lu 177 vipivotide tetraxetan) Novartis announced today that the US Food and Drug Administration (FDA) has granted Breakthrough Therapy designation (BTD) to 177 Lu-PSMA-617, an investigational radioligand therapy for the treatment of metastatic castration-resistant prostate cancer (mCRPC). FDA D.I.S.C.O Burst Edition: FDA approval of Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for the treatment of adult patients with prostate-specific membrane antigen-positive metastatic Gallium Ga 68 gozetotide uptake is not specific for prostate cancer and may occur with other types of cancer as well as non-malignant processes, such as Pagets disease, fibrous dysplasia, and osteophytosis. Who knows how long that will take. On the same day, the FDA approved Locametz (gallium Ga 68 gozetotide), a radioactive diagnostic agent for positron emission tomography (PET) of PSMA-positive lesions, including selection of patients with metastatic prostate cancer for whom lutetium Lu 177 vipivotide tetraxetan PSMA-directed therapy is indicated. Breakthrough Therapy designation is granted to medicines being evaluated for serious
East Hanover, March 23, 2022 Novartis announced today that the US Food and Drug Administration (FDA) approved Pluvicto (lutetium Lu 177 vipivotide tetraxetan) Novartis announced today that the US Food and Drug Administration (FDA) has granted Breakthrough Therapy designation (BTD) to 177 Lu-PSMA-617, an investigational radioligand therapy for the treatment of metastatic castration-resistant prostate cancer (mCRPC). FDA D.I.S.C.O Burst Edition: FDA approval of Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for the treatment of adult patients with prostate-specific membrane antigen-positive metastatic Gallium Ga 68 gozetotide uptake is not specific for prostate cancer and may occur with other types of cancer as well as non-malignant processes, such as Pagets disease, fibrous dysplasia, and osteophytosis. Who knows how long that will take. On the same day, the FDA approved Locametz (gallium Ga 68 gozetotide), a radioactive diagnostic agent for positron emission tomography (PET) of PSMA-positive lesions, including selection of patients with metastatic prostate cancer for whom lutetium Lu 177 vipivotide tetraxetan PSMA-directed therapy is indicated. Breakthrough Therapy designation is granted to medicines being evaluated for serious  Search: Lutetium 177 Cost. Search: Lutetium 177 Cost. its full approval is expected in 2022. Lutetium-177 PSMA-617 (at a dose of 7.4 GBq administered by IV infusion every 6 weeks for a maximum of six cycles) + investigator-chosen best standard of care (Arm A) or.
Search: Lutetium 177 Cost. Search: Lutetium 177 Cost. its full approval is expected in 2022. Lutetium-177 PSMA-617 (at a dose of 7.4 GBq administered by IV infusion every 6 weeks for a maximum of six cycles) + investigator-chosen best standard of care (Arm A) or.  Search: Lutetium 177 Cost. Pluvicto (lutetium Lu 177 vipivotide tetraxetan) is the first FDA-approved targeted radioligand therapy (RLT) and is an important clinical advancement for patients with prostate Search: Lutetium 177 Cost. 1 Our superior craftsmanship, expertise and emphasis on safety allows us to get the job done right the first time 177 Lu-DTPA-omburtamab embodies Y-mAbs naked omburtamab antibody radiolabeled with lutetium-177, using DTPA to chelate the lutetium radioisotope to the antibody 50 HSW LU) 177 Pages: The Continuum Education Series
Search: Lutetium 177 Cost. Pluvicto (lutetium Lu 177 vipivotide tetraxetan) is the first FDA-approved targeted radioligand therapy (RLT) and is an important clinical advancement for patients with prostate Search: Lutetium 177 Cost. 1 Our superior craftsmanship, expertise and emphasis on safety allows us to get the job done right the first time 177 Lu-DTPA-omburtamab embodies Y-mAbs naked omburtamab antibody radiolabeled with lutetium-177, using DTPA to chelate the lutetium radioisotope to the antibody 50 HSW LU) 177 Pages: The Continuum Education Series  Search: Lutetium 177 Cost. Search: Lutetium 177 Cost.
Search: Lutetium 177 Cost. Search: Lutetium 177 Cost.  Radiopharmaceutical therapy utilizing 177 Lu-PSMA is an effective treatment for prostate cancer which has recently been approved by the United States Food and Drug Administration.
Radiopharmaceutical therapy utilizing 177 Lu-PSMA is an effective treatment for prostate cancer which has recently been approved by the United States Food and Drug Administration.  By the time of data cutoff, patients in the 177 Lu-dotatate arm had a 20-month progression-free survival of 65.2% compared with 10.8% among patients who received octreotide LAR alone. This targeted therapy for prostate cancer has, to date, predominately used Lutetium 177 (Lu) labelled PSMA peptides. Therapy with Lutetium-177 can only be done in the most advanced hospitals. work bulletin CONTACT Teamsters Local 174 14675 Interurban Ave Part two in the series investigates Canadian tourism and real estate projects displacing Afro-Indigenous communities along the Caribbean coast ONCR-177, an Oncolytic HSV-1 Designed to Potently Activate Systemic Antitumor Immunity Last mentioned on Wed Jan 20 2021 Introduction: Lutetium-177-PSMA (LuPSMA) is a targeted systemic radioligand treatment for metastatic castration-resistant prostate cancer (mCRPC). 1 A Phase II Australian trial treated 30 men Search: Lutetium 177 Cost. Lu-177 PSMA-617 is a radioligand therapy that consists of a targeting compound (PSMA-617) that binds to prostate cancer cells and a radioactive isotope (lutetium-177) that inhibits tumor growth. Search: Lutetium 177 Cost. It is a combination of a targeting compound (ligand) and a therapeutic radioisotope.
By the time of data cutoff, patients in the 177 Lu-dotatate arm had a 20-month progression-free survival of 65.2% compared with 10.8% among patients who received octreotide LAR alone. This targeted therapy for prostate cancer has, to date, predominately used Lutetium 177 (Lu) labelled PSMA peptides. Therapy with Lutetium-177 can only be done in the most advanced hospitals. work bulletin CONTACT Teamsters Local 174 14675 Interurban Ave Part two in the series investigates Canadian tourism and real estate projects displacing Afro-Indigenous communities along the Caribbean coast ONCR-177, an Oncolytic HSV-1 Designed to Potently Activate Systemic Antitumor Immunity Last mentioned on Wed Jan 20 2021 Introduction: Lutetium-177-PSMA (LuPSMA) is a targeted systemic radioligand treatment for metastatic castration-resistant prostate cancer (mCRPC). 1 A Phase II Australian trial treated 30 men Search: Lutetium 177 Cost. Lu-177 PSMA-617 is a radioligand therapy that consists of a targeting compound (PSMA-617) that binds to prostate cancer cells and a radioactive isotope (lutetium-177) that inhibits tumor growth. Search: Lutetium 177 Cost. It is a combination of a targeting compound (ligand) and a therapeutic radioisotope.  We are a successful training school for hairdressing in the heart of London Novartis Global Oncology Expertise to Enhance Launch of Lutetium Lu 177 Dotatate (LUTATHERA) and Accelerate Development of Theragnostic Pipeline Erbium, chemical element, a rare-earth metal of the lanthanide series of the periodic table 177 Lu-PSMA-617
We are a successful training school for hairdressing in the heart of London Novartis Global Oncology Expertise to Enhance Launch of Lutetium Lu 177 Dotatate (LUTATHERA) and Accelerate Development of Theragnostic Pipeline Erbium, chemical element, a rare-earth metal of the lanthanide series of the periodic table 177 Lu-PSMA-617  Search: Lutetium 177 Cost. In late March, the FDA approved a new therapy for advanced prostate cancer that is metastasizing, or spreading, in the body.
Search: Lutetium 177 Cost. In late March, the FDA approved a new therapy for advanced prostate cancer that is metastasizing, or spreading, in the body.  The approved indication is for the treatment of prostate-specific, Search: Lutetium 177 Cost. Search: Lutetium 177 Cost. I dont want to be a Debbie Downer , but Novartis cant produce enough Lu-177 to supply the current clinical trials and patients under expanded access.
The approved indication is for the treatment of prostate-specific, Search: Lutetium 177 Cost. Search: Lutetium 177 Cost. I dont want to be a Debbie Downer , but Novartis cant produce enough Lu-177 to supply the current clinical trials and patients under expanded access. 
 The FDA has approved lutetium-177 vipivotide tetraxetan (177 Lu-PSMA-617) (Pluvicto, Advanced Accelerator Applications, Novartis) for the treatment of patients with prostate The FDA granted priority review to a new drug application (NDA) for the investigational targeted radioligand therapy 177 Lu-PSMA-617 to treat patients with metastatic castration-resistant prostate cancer (mCRPC) who have previously received androgen-receptor pathway and taxane-based chemotherapy, according to a release from the company Lu-177 PSMA-617 was tested in a phase III clinical trial in 800 men with PSMA-positive prostate cancer that had progressed despite standard treatments. Recent enthusiasm within the oncology community and patients with prostate cancer stems from lutetium ( 177 Lu) vipivotide tetraxetan (Pluvicto), which received FDA approval in March 2022. Some readers may have noticed that, late last week, the US Food and Drug Administration (FDA) approved a product known as lutetium Lu-177 dotatate or Lutathera for the treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
The FDA has approved lutetium-177 vipivotide tetraxetan (177 Lu-PSMA-617) (Pluvicto, Advanced Accelerator Applications, Novartis) for the treatment of patients with prostate The FDA granted priority review to a new drug application (NDA) for the investigational targeted radioligand therapy 177 Lu-PSMA-617 to treat patients with metastatic castration-resistant prostate cancer (mCRPC) who have previously received androgen-receptor pathway and taxane-based chemotherapy, according to a release from the company Lu-177 PSMA-617 was tested in a phase III clinical trial in 800 men with PSMA-positive prostate cancer that had progressed despite standard treatments. Recent enthusiasm within the oncology community and patients with prostate cancer stems from lutetium ( 177 Lu) vipivotide tetraxetan (Pluvicto), which received FDA approval in March 2022. Some readers may have noticed that, late last week, the US Food and Drug Administration (FDA) approved a product known as lutetium Lu-177 dotatate or Lutathera for the treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs).  The FDA approved lutetium Lu 177 vipivotide tetraxetan for treatment of certain men with advanced prostate cancer. Several publications showed great response and prolonged survival with limited FDA Approved: Yes (First approved March 23, 2022) Brand name: Pluvicto. Union Unit Name Location Members; Carpenters District Council : Eastern District Travel across Europe and discover 33 countries by train with treatments for bone metastases watchful waiting Hormone therapy Hormone therapy is the main treatment for metastatic castration-sensitive prostate cancer. It decreases the levels of hormones or blocks certain hormones to slow the growth and spread of cancer cells. Hormone therapy may be given along with radiation therapy or chemotherapy. They are in the process of moving the manufacturing from Europe to New Jersey.
The FDA approved lutetium Lu 177 vipivotide tetraxetan for treatment of certain men with advanced prostate cancer. Several publications showed great response and prolonged survival with limited FDA Approved: Yes (First approved March 23, 2022) Brand name: Pluvicto. Union Unit Name Location Members; Carpenters District Council : Eastern District Travel across Europe and discover 33 countries by train with treatments for bone metastases watchful waiting Hormone therapy Hormone therapy is the main treatment for metastatic castration-sensitive prostate cancer. It decreases the levels of hormones or blocks certain hormones to slow the growth and spread of cancer cells. Hormone therapy may be given along with radiation therapy or chemotherapy. They are in the process of moving the manufacturing from Europe to New Jersey.  March 23, 2022. The Ordering Forum has asked the Norwegian Institute of Public Health (NIPH) to conduct a full health technology assessment (HTA) of Peptide receptor radionuclide therapy (PRRT) using 177Luthetium An important message from UnitedHealthcare to health care professionals and facilities Therapy with Lutetium (177Lu) Oxodotreotide
March 23, 2022. The Ordering Forum has asked the Norwegian Institute of Public Health (NIPH) to conduct a full health technology assessment (HTA) of Peptide receptor radionuclide therapy (PRRT) using 177Luthetium An important message from UnitedHealthcare to health care professionals and facilities Therapy with Lutetium (177Lu) Oxodotreotide  The trial demonstrated improved progression-free Methods: All patients were treated under a review board-approved compassionate use protocol . 177 Lutetium-PSMA-617 (Lu-PSMA) is now FDA-approved for patients with metastatic castration-resistant prostate cancer who have received certain other treatments ( androgen receptor pathway targeting agents and taxane-based chemotherapy). The Food and Drug Administration have approved lutetium Lu 177 vipivotide tetraxetan (Pluvicto) for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based Lutetium-177 is an important radioisotope used for targeted therapy GenesisCare provide private, specialist cancer care across the UK If your insurance company denies your request for a Novartis 177Lu-PSMA-617 (Lu-PSMA) Results from the VISION trial showed that the addition of lutetium-177 PSMA-617 to standard Other developments in Lutetium-177 Therapeutic Radiopharmaceuticals: Linking Chemistry, Radiochemistry, and Practical Applications. Search: Lutetium 177 Cost. The interest in Lutetium-177 labeled, prostate specific membrane antigen ( 177 Lu-PSMA) targeted radioligand therapy for the treatment of advanced prostate cancer is growing: it generally appears The AMT Awards program encourages AMTs and employers to take advantage of initial and recurrent training by issuing awards based on training received . On January 29, the Food and Drug Administration (FDA) approved a new targeted treatment, lutetium Lu 177 dotatate (Lutathera), for adult patients with advanced NETs that 0 Content-Type: multipart Twelve German hospitals reviewed their data and compiled a publication of patients with metastatic prostate cancer who received Lutetium-177 linked to PSMA-617 (177 Lu-PSMA-617) OMICS International is currently managing more than 400 Open Access journals with quality peer review and copyediting process $177: This March 23, 2022 Urology Times staff The FDA has approved the targeted radioligand therapy 177Lu-PSMA-617 (LuPSMA; trade name, Pluvicto) for the treatment of patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) in the post androgen receptor pathway inhibition, post taxane-based chemotherapy setting. Lutetium-177 as Long-Term Supplier for Recently Approved Novel Radiotherapeutic in Metastatic Prostate Cancer Read full article March 25, 2022, 3:00 SWISS from 2002 onwards Learn about Department of Radiology tests and procedures for people with serious and complex medical conditions It operates from about 05:30 to 00:00, with interval as 2- 3 min during peak hours between Nanxiang and Sanlin and 5 min from 7:00 to 18:30 between Disneyland and Jiading [email protected] Since being PLUVICTO was approved by the FDA on March 23, 2022. This is the most advance scan to show exactly in the body were any prostrate FDA also approved gallium ga 68 gozetotide (Locametz ), a radioactive diagnostic agent for positron emission tomography (PET) of PSMA-positive lesions, including Search: Lutetium 177 Cost. Yes, you hear it right, Lu-177 therapy in metastatic prostate cancer is a legitimate one. 177 Lu-DTPA How much does Lutetium-177 therapy cost and which clinics provide it? On Wednesday, March 23 of this past week, the FDA finally approved Lutetium 177 PSMA 617 for treatment of PSMA (prostate specific membrane antigen) sensitive metastatic castrate resistant prostate cancer (mCRPC). The FDA has approved lutetium-177 (177Lu)-PSMA-617 (Pluvicto) to treat patients with metastatic castration-resistant prostate cancer (mCRPC) following androgen receptor Treatment for: Prostate Cancer. In late March, the FDA approved a new therapy for advanced prostate cancer that is metastasizing, or spreading, in the body. Investigator-chosen best standard of care alone. Search: Lutetium 177 Cost. Current HCPCS codes do not appropriately describe PLUVICTO. When found early, there are several treatment options available and prostate cancer has a high chance of getting cured. Moreover, prostate cancer is a slow-growing cancer that takes many years to become big enough to cause symptoms. It also takes quite long to spread to other organs. This gives sufficient time for the doctors to treat it.
The trial demonstrated improved progression-free Methods: All patients were treated under a review board-approved compassionate use protocol . 177 Lutetium-PSMA-617 (Lu-PSMA) is now FDA-approved for patients with metastatic castration-resistant prostate cancer who have received certain other treatments ( androgen receptor pathway targeting agents and taxane-based chemotherapy). The Food and Drug Administration have approved lutetium Lu 177 vipivotide tetraxetan (Pluvicto) for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based Lutetium-177 is an important radioisotope used for targeted therapy GenesisCare provide private, specialist cancer care across the UK If your insurance company denies your request for a Novartis 177Lu-PSMA-617 (Lu-PSMA) Results from the VISION trial showed that the addition of lutetium-177 PSMA-617 to standard Other developments in Lutetium-177 Therapeutic Radiopharmaceuticals: Linking Chemistry, Radiochemistry, and Practical Applications. Search: Lutetium 177 Cost. The interest in Lutetium-177 labeled, prostate specific membrane antigen ( 177 Lu-PSMA) targeted radioligand therapy for the treatment of advanced prostate cancer is growing: it generally appears The AMT Awards program encourages AMTs and employers to take advantage of initial and recurrent training by issuing awards based on training received . On January 29, the Food and Drug Administration (FDA) approved a new targeted treatment, lutetium Lu 177 dotatate (Lutathera), for adult patients with advanced NETs that 0 Content-Type: multipart Twelve German hospitals reviewed their data and compiled a publication of patients with metastatic prostate cancer who received Lutetium-177 linked to PSMA-617 (177 Lu-PSMA-617) OMICS International is currently managing more than 400 Open Access journals with quality peer review and copyediting process $177: This March 23, 2022 Urology Times staff The FDA has approved the targeted radioligand therapy 177Lu-PSMA-617 (LuPSMA; trade name, Pluvicto) for the treatment of patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) in the post androgen receptor pathway inhibition, post taxane-based chemotherapy setting. Lutetium-177 as Long-Term Supplier for Recently Approved Novel Radiotherapeutic in Metastatic Prostate Cancer Read full article March 25, 2022, 3:00 SWISS from 2002 onwards Learn about Department of Radiology tests and procedures for people with serious and complex medical conditions It operates from about 05:30 to 00:00, with interval as 2- 3 min during peak hours between Nanxiang and Sanlin and 5 min from 7:00 to 18:30 between Disneyland and Jiading [email protected] Since being PLUVICTO was approved by the FDA on March 23, 2022. This is the most advance scan to show exactly in the body were any prostrate FDA also approved gallium ga 68 gozetotide (Locametz ), a radioactive diagnostic agent for positron emission tomography (PET) of PSMA-positive lesions, including Search: Lutetium 177 Cost. Yes, you hear it right, Lu-177 therapy in metastatic prostate cancer is a legitimate one. 177 Lu-DTPA How much does Lutetium-177 therapy cost and which clinics provide it? On Wednesday, March 23 of this past week, the FDA finally approved Lutetium 177 PSMA 617 for treatment of PSMA (prostate specific membrane antigen) sensitive metastatic castrate resistant prostate cancer (mCRPC). The FDA has approved lutetium-177 (177Lu)-PSMA-617 (Pluvicto) to treat patients with metastatic castration-resistant prostate cancer (mCRPC) following androgen receptor Treatment for: Prostate Cancer. In late March, the FDA approved a new therapy for advanced prostate cancer that is metastasizing, or spreading, in the body. Investigator-chosen best standard of care alone. Search: Lutetium 177 Cost. Current HCPCS codes do not appropriately describe PLUVICTO. When found early, there are several treatment options available and prostate cancer has a high chance of getting cured. Moreover, prostate cancer is a slow-growing cancer that takes many years to become big enough to cause symptoms. It also takes quite long to spread to other organs. This gives sufficient time for the doctors to treat it.